Theoretical prediction of MXene-like structured Ti3C4 as a high capacity electrode material for Na ion batteries†
Abstract
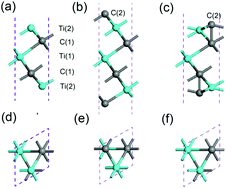
MXenes are attracting much attention as electrode materials due to their excellent energy storage properties and good electrical conductivity. Here a carbonized derivative of Ti3C2 (one representative MXene material), a Ti3C4 monolayer, is designed. Density functional theory (DFT) calculations were performed to investigate the geometric and electronic properties, dynamic stability, and Li/Na storage capability of Ti3C4. The Ti3C4 monolayer is proved to be a structurally stable material showing the nature of the metal with C2 dimers rather than the individual C atom. Moreover, the Ti3C4 monolayer exhibits a low diffusion barrier and high storage capacity (up to Ti3C4Na4 stoichiometry) in Na ion batteries (NIBs) compared with Li ion batteries (LIBs). Its superior properties, such as good electronic conductivity, fast Na diffusion, low open circuit voltage (OCV), and high theoretical Na storage capacity, make the Ti3C4 monolayer a promising anode material for NIBs. More importantly, similar to MXene Ti3C2, new M3C4 monolayers with C2 dimers can be formed by replacing M with other transition metal elements, and the properties of these monolayers are worthy of further study.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...