Suppressing the effect of lithium dendritic growth by the addition of magnesium bis(trifluoromethanesulfonyl)amide†
Abstract
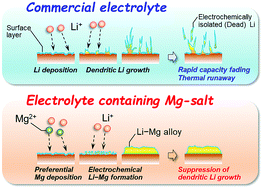
Practical applications of Li–S and Li–air batteries require the morphology of the Li metal negative electrode during charge/discharge (i.e., Li-deposition/dissolution) cycling to be precisely controlled. Herein, we used magnesium bis(trifluoromethanesulfonyl)amide [Mg(TFSA)2] as an electrolyte additive to suppress the growth of Li dendrites, utilizing the occurrence of an alloying reaction between the initially substrate-deposited Mg and the subsequently deposited Li. Notably, no metallic Mg formation and no change in Li deposition morphology were observed at an electrolyte composition of 0.1 M Mg(TFSA)2 + 0.9 M LiTFSA/triglyme, irrespective of the applied potential. In contrast, increasing the Mg salt concentration to 0.5 M resulted in the deposition of interconnected granules, reflecting a dramatic morphology improvement. X-ray diffraction analysis revealed the occurrence of the abovementioned alloying, which finally afforded a deposit composition of Li0.9Mg0.1via the formation of an intermediate Li0.14Mg0.86 phase. Importantly, the deposits obtained under various applied potentials were relatively smooth, with no needle-like morphology observed.


 Please wait while we load your content...
Please wait while we load your content...