Formation of spherical ice-shells inside carbon fullerenes
Abstract
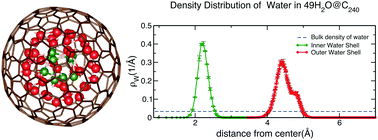
The structural and dynamic properties of encapsulated water inside fullerene cages, C60 to C320, were investigated employing classical molecular dynamics simulations. We find that the confined water forms single to multiple concentric, spherical shells as the size of the fullerene increases. This is possible due to the reduced number of hydrogen bonds per water molecule in the nanoscale liquid as compared to bulk water, allowing the encapsulated H2O molecules to imitate the shape of the confining boundary. These water-cluster shells exhibit solid-like behavior at temperatures as high as 500 K. Our current findings complement the existing literature on water confined by sp2-hybridized nanocarbon structures including one dimensional nanotubes and two dimensional graphene sheets.


 Please wait while we load your content...
Please wait while we load your content...