Hidden complexities in the reaction of H2O2 and HNO revealed by ab initio quantum chemical investigations†
Abstract
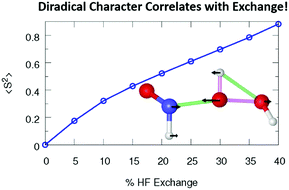
Nitroxyl (HNO) and hydrogen peroxide have both been implicated in a variety of reactions relevant to environmental and physiological processes and may contribute to a unique, unexplored, pathway for the production of nitrous acid (HONO) in soil. To investigate the potential for this reaction, we report an in-depth investigation of the reaction pathway of H2O2 and HNO forming HONO and water. We find the breaking of the peroxide bond and a coupled proton transfer in the first step leads to hydrogen nitryl (HNO2) and an endogenous water, with an extrapolated NEVPT2 (multireference perturbation theory) barrier of 29.3 kcal mol−1. The first transition state is shown to possess diradical character linking the far peroxide oxygen to the bridging, reacting, peroxide oxygen. The energy of this first step, when calculated using hybrid density functional theory, is shown to depend heavily on the amount of Hartree–Fock exchange in the functional, with higher amounts leading to a higher barrier and more diradical character. Additionally, high amounts of spin contamination cause CCSD(T) to significantly overestimate the TS1 barrier with a value of 36.2 kcal mol−1 when using the stable UHF wavefunction as the reference wavefunction. However, when using the restricted Hartree–Fock reference wavefunction, the TS1 CCSD(T) energy is lowered to yield a barrier of 31.2 kcal mol−1, in much better agreement with the NEVPT2 result. The second step in the reaction is the isomerization of HNO2 to trans-HONO through a Grotthuss-like mechanism accepting a proton from and donating a proton to the endogenous water. This new mechanism for the isomerization of HNO2 is shown to have an NEVPT2 barrier of 23.3 kcal mol−1, much lower than previous unimolecular estimates not including an explicit water. Finally, inclusion of an additional explicit water is shown to lower the HNO2 isomerization barrier even further.


 Please wait while we load your content...
Please wait while we load your content...