Unravelling the hydrophobicity of urea in water using thermodiffusion: implications for protein denaturation†
Abstract
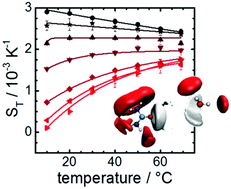
Urea is widely used as a protein denaturant in aqueous solutions. Experimental and computer simulation studies have shown that it dissolves in water almost ideally at high concentrations, introducing little disruption in the water hydrogen bonded structure. However, at concentrations of the order of 5 M or higher, urea induces denaturation in a wide range of proteins. The origin of this behaviour is not completely understood, but it is believed to stem from a balance between urea–protein and urea–water interactions, with urea becoming possibly hydrophobic at a specific concentration range. The small changes observed in the water structure make it difficult to connect the denaturation effects to the solvation properties. Here we show that the exquisite sensitivity of thermodiffusion to solute–water interactions allows the identification of the onset of hydrophobicity of urea–water mixtures. The hydrophobic behaviour is reflected in a sign reversal of the temperature dependent slope of the Soret coefficient, which is observed, both in experiments and non-equilibrium computer simulations at ∼5 M concentration of urea in water. This concentration regime corresponds to the one where abrupt changes in the denaturation of proteins are commonly observed. We show that the onset of hydrophobicity is intrinsically connected to the urea–water interactions. Our results allow us to identify correlations between the Soret coefficient and the partition coefficient, log P, hence establishing the thermodiffusion technique as a powerful approach to study hydrophobicity.


 Please wait while we load your content...
Please wait while we load your content...