Structural origins of the cohesive energy in metal-terpyridine oligomer thin-films†
Abstract
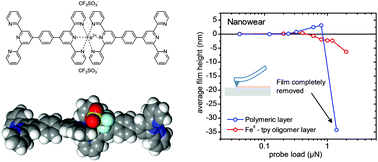
FeII-terpyridine based oligomers have attracted considerable interest as key constituents for the realization of highly robust, ultra-thin ordered layers of metal center oligomers (MCOs) for organic electronics applications. By using molecular simulations and nanotribology investigations, we report on the origins of the surprisingly high mechanical and thermal stability in this type of MCO layers, which finds its expression in nanowear resistance values of up to 1.5 μN for the MCO films, as well as in a thermal stability of two-terminal MCO junctions to temperatures up to ∼100 °C under electrical load. A theoretical analysis of the fundamental cohesive forces among the constituents within the context of an electrostatic model reveal that the cohesive energy is essentially based on Coulomb interactions among the ionic constituents of the oligomers, leading to an estimated cohesive energy per molar mass of 0.0132 eV mol g−1 for MCO layers that advantageously compare to the 0.0061 eV mol g−1 reported for pentacene crystals.


 Please wait while we load your content...
Please wait while we load your content...