The electronic structure of Ag1−xSn1+xSe2 (x = 0.0, 0.1, 0.2, 0.25 and 1.0)
Abstract
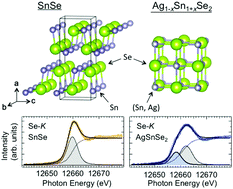
We have studied the valence electronic structure of Ag1−xSn1+xSe2 (x = 0.0, 0.1, 0.2, 0.25) and SnSe (x = 1.0) by a combined analysis of X-ray absorption spectroscopy (XAS) and X-ray photoemission spectroscopy (XPS) measurements. Both XAS and XPS reveal an increase in electron carriers in the system with x (i.e. excess Sn concentration) for 0 ≤ x ≤ 0.25. The core-level spectra (Sn 3d, Ag 3d and Se 3d) show that the charge state of Ag is almost 1+, while that of of Sn splits into Sn2+ and Sn4+ (providing clear evidence of valence skipping for the first time) with a concomitant splitting of Se into Se2− and Se2−δ states. The x dependence of the split components in Sn and Se together with the Se-K edge XAS reveals that the Se valence state may have an essential role in the transport properties of this system.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...