The molecular mechanism of Nystatin action is dependent on the membrane biophysical properties and lipid composition†
Abstract
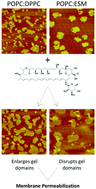
Nystatin (Nys) is a pore forming broad-spectrum and efficient antifungal drug with significant toxicity in mammalian organisms. In order to develop a non-toxic and more effective Nys formulation, its molecular mechanism of action at the cell membrane needs to be better understood. It is widely accepted that Nys activity and toxicity depend on the presence and type of membrane sterols. Taking advantage of multiple biophysical methodologies, we now show that the formation and stabilization of Nys aqueous pores, which are associated with Nys cytotoxicity, occur in the absence of membrane sterols. Our results suggest that the Nys mechanism of action is driven by the presence of highly ordered membrane domains capable of stabilizing the Nys oligomers. Moreover, Nys pore formation is accompanied by strong Nys-induced membrane reorganization that depends on membrane lipid composition and seems to underlie the Nys cytotoxic effect. Accordingly, in membranes enriched in a gel-phase forming phospholipid, Nys incorporates within the phospholipid-enriched gel domains, where it forms pores able to expand the gel domains. In contrast, in membranes enriched in gel domain forming sphingolipids, Nys-induced pore formation occurs through the destabilization of the gel phase. These results show that the Nys mechanism of action is complex and not only dependent on membrane sterols, and provide further insight into the molecular details governing Nys activity and toxicity.


 Please wait while we load your content...
Please wait while we load your content...