Structural rationale for the chiral separation and migration order reversal of clenpenterol enantiomers in capillary electrophoresis using two different β-cyclodextrins†
Abstract
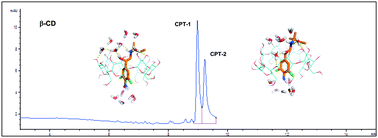
NMR spectroscopy experiments, molecular dynamics simulations, and theoretical chemistry calculations provide insight into the structural and energetic determinants of the distinct binding of clenpenterol enantiomers to two cyclodextrins and the migration order reversal of their respective inclusion complexes in capillary electrophoresis.


 Please wait while we load your content...
Please wait while we load your content...