Two-dimensional GeP3 as a high capacity electrode material for Li-ion batteries†
Abstract
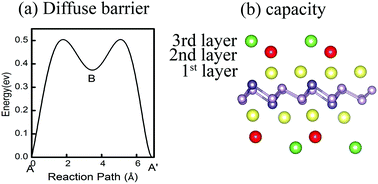
Two-dimensional (2D) materials are promising for use in lithium (Li) electrodes due to their high surface ratio. By using density functional theory (DFT) calculations, we investigate the adsorption and diffusion of Li on a newly predicted 2D GeP3 material [Nano Lett., 2016, 17, 1833]. The most favourable adsorption sites for Li are identified, and a semiconducting to metallic transition induced by Li adsorption is found, which indicates excellent electrical conductivity. The GeP3 monolayer has an estimated capacity of 648 mA h g−1, which is almost twice that of commercially used graphite (375 mA h g−1). During full Li intercalation, the GeP3 layer undergoes only 1.2% lattice parameter reduction. Moreover, GeP3 possesses the advantages of a small diffusion barrier (∼0.5 eV) and low average open-circuit voltages (∼0.4 V). Our results highlight a new class of promising anode materials, i.e. 2D phosphide, as potential rechargeable lithium batteries with ultrahigh-capacity, superior ionic conductivity, and low average open-circuit voltage.


 Please wait while we load your content...
Please wait while we load your content...