Surface pKa of octanoic, nonanoic, and decanoic fatty acids at the air–water interface: applications to atmospheric aerosol chemistry†
Abstract
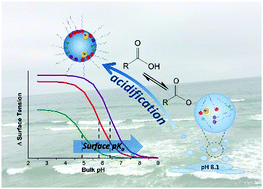
There exists large uncertainty in the literature as to the pKa of medium-chain fatty acids at the air–water interface. Via surface tension titration, the surface-pKa values of octanoic (C8), nonanoic (C9), and decanoic (C10) fatty acids are determined to be 4.9, 5.8, and 6.4, respectively. The surface-pKa determined with surface tension differs from the bulk value obtained during a standard acid–base titration. Near the surface-pKa of the C8 and C9 systems, surface tension minima are observed and are attributed to the formation of surface-active acid–soap complexes. The direction of the titration is shown to affect the surface-pKa of the C9 system, as the value shifts to 5.2 with NaOH titrant due to a higher concentration of Na+ ions at pH values close to the surface-pKa. As the reactivity and climate-relevant properties of sea spray aerosols (SSA) are partially dictated by the charge and surface activity of the organics at the aerosol–atmosphere interface, the results presented here on SSA-identified C8–C10 fatty acids can be used to better predict the health and climate impact of particles with significant concentrations of medium-chain fatty acids.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...