Proof of ion-pair structures in ammonium-based protic ionic liquids using combined NMR and DFT/PCM-based chemical shift calculations†
Abstract
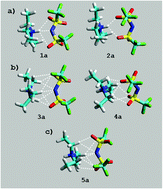
The self-assembly of triethylammonium bis(trifluoromethylsulfonyl)imide, i.e. [(C2H5)3NH][TFSI], in chloroform and aqueous solutions has been investigated using 1H NMR spectroscopy and computational (DFT/PCM prediction) methods. We have examined a number of ion pairs formed between the [(C2H5)3NH]+ cation with different conformations of alkyl substituents as well as various dispositions of the multi-site [TFSI]− anion. Based on the agreement between the calculated (DFT) and observed 1H NMR chemical shifts, [(C2H5)3NH][TFSI] in chloroform formed lipophilic complexes with effective N+–H⋯N or N+–H⋯O hydrogen bonding, whereas hydrophilic complexes with Cα–H⋯O and Cα–H⋯F hydrogen bonding are found in aqueous solutions. This study provides a new insight into the self-aggregation of ammonium PILs incorporating the widely used [TFSI]− anion and demonstrates the importance of solvent effects on chemical shifts. The simulations with explicit and implicit dielectric continuum solvents are found to be the most realistic method, yielding a representative ensemble of structures.


 Please wait while we load your content...
Please wait while we load your content...