DFT coupled with NEGF study of ultra-sensitive HCN and HNC gases detection and distinct I–V response based on phosphorene†
Abstract
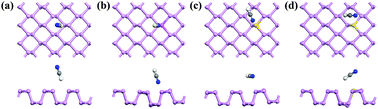
The sensing performances of pristine and X-doped phosphorene substrates (X = Al, Si, and S atoms) toward the adsorption of the toxic gases HCN and HNC were systematically investigated by first-principles simulations. The numerical results show that the pristine phosphorene is sensitive to HCN and HNC molecules with moderate adsorption energy, excellent charge transfer, high sensitivity and selectivity, implying its potential applications as excellent HCN and HNC sensors. In addition, the Al-doped phosphorene exhibits extremely high reactive activity toward HCN and HNC gases; thus, it has potential for use as a metal-free catalyst for activating or catalyzing HCN or HNC adsorbates. Moreover, the transport properties, i.e., current–voltage (I–V) characteristics, were calculated by the non-equilibrium Green's function (NEGF) method within the framework of the density functional theory (DFT). The obtained results reveal that the adsorbed HCN or HNC gas molecules have a remarkable impact on the electronic conductivity of phosphorene, and the zigzag direction of phosphorene is more sensitive to gas molecules than the armchair direction. The combination of the high sensitivity, superior selectivity, and moderate adsorption energy of pristine phosphorene toward HCN or HNC gas molecules adsorption, makes phosphorene an excellent candidate for HCN and HNC sensors.


 Please wait while we load your content...
Please wait while we load your content...