SO3 formation from the X-ray photolysis of SO2 astrophysical ice analogues: FTIR spectroscopy and thermodynamic investigations
Abstract
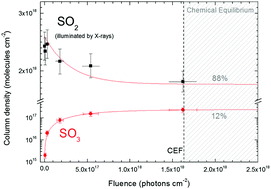
In this combined experimental–theoretical work we focus on the physical and chemical changes induced by soft X-rays on sulfur dioxide (SO2) ice at a very low temperature, in an attempt to clarify and quantify its survival and chemical changes in some astrophysical environments. SO2 is an important constituent of some Jupiter moons and has also been observed in ices around protostars. The measurements were performed at the Brazilian Synchrotron Light Source (LNLS/CNPEM), in Campinas, Brazil. The SO2 ice sample (12 K) was exposed to a broadband beam of mainly soft X-rays (6–2000 eV) and in situ analyses were performed by IR spectroscopy. The X-ray photodesorption yield (upper limit) was around 0.25 molecules per photon. The values determined for the effective destruction (SO2) and formation (SO3) cross sections were 2.5 × 10−18 cm2 and 2.1 × 10−18 cm2, respectively. The chemical equilibrium (88% of SO2 and 12% of SO3) was reached after the fluence of 1.6 × 1018 photons cm−2. The SO3 formation channels were studied at the second-order Møller–Plesset perturbation theory (MP2) level, which showed the three most favorable reaction routes (ΔH < −79 kcal mol−1) in simulated SO2 ice: (i) SO + O2 → SO3, (ii) SO2 + O → SO3, and (iii) SO2 + O+ → SO3+ + e− → SO3. The amorphous solid environment effect decreases the reactivity of intermediate species towards SO3 formation, and ionic species are even more affected. The experimentally determined effective cross sections and theoretical reaction channels identified in this work allow us to better understand the chemical evolution of certain sulfur-rich astrophysical environments.


 Please wait while we load your content...
Please wait while we load your content...