Photoexcitation dynamics of p-nitroaniline and N,N-dimethyl-p-nitroaniline in 1-alkyl-3-methylimidazolium-cation based ionic liquids with different alkyl-chain lengths†
Abstract
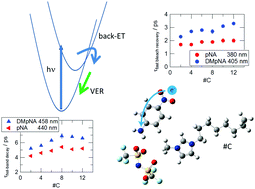
Photoexcitation dynamics of p-nitroaniline (pNA) and N,N-dimethyl-p-nitroaniline (DMpNA) in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([Cnmim][NTf2]) with different alkyl chain lengths (from C2 to C12) was investigated using transient absorption spectroscopy. The internal conversion rate from the excited state to the ground state was estimated from bleach recovery around the ground state absorption centre, and the successive vibrational cooling rate in the ground state was estimated from the decay of the hot band observed at the red-edge of ground state absorption. The internal conversion rate slightly decreased with an increase in the alkyl-chain length of the cation, while the dependence of DMpNA was more significant than that of pNA. The extent of change was correlated with the change of the reaction free energy and solvent reorganization energy estimated from the absorption spectrum assuming that the internal conversion process is modelled by a back-electron-transfer process. The vibrational cooling rate estimated from the decay of hot-band absorption slightly decreased with an increase in the alkyl-chain length of the cation for both solutes. The hot-band decay of pNA was about 1.5-times faster than that of DMpNA, irrespective of the alkyl-chain length.


 Please wait while we load your content...
Please wait while we load your content...