The opening/closure of the P-loop and hinge of BCR-ABL1 decodes the low/high bioactivities of dasatinib and axitinib†
Abstract
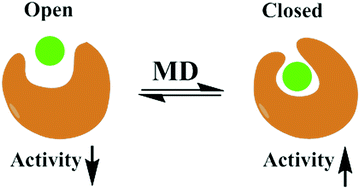
To obtain new insights into the resistance caused by T315I and the differential selectivities of dasatinib and axitinib against BCR-ABL1(WT) and BCR-ABL1(T315), molecular dynamics simulations and free energy calculations were performed. A rule is summarized that the opening/closure of the P-loop and hinge of BCR-ABL1 could reflect the low/high bioactivities of dasatinib and axitinib. This may be because strong interactions of the ligands with key residues induce the P-loop and hinge of BCR-ABL1 to close, being favorable for the retention of the ligand in the binding site. However, weak interactions of the ligands with key residues cause the P-loop and hinge of BCR-ABL1 to open, improving the dissociation of the ligand. These key residues, which predominantly govern the differential selectivities of dasatinib and axitinib against BCR-ABL1(WT) and BCR-ABL1(T315), are highlighted, and the impacts of T315 mutation and the chemical structures of dasatinib and axitinib are also explored. The rule extracted from this study may have potential in screening kinase inhibitors, rationally designing the next generation kinase inhibitors, and guiding the precise treatment of kinase-related cancer.


 Please wait while we load your content...
Please wait while we load your content...