Thermodynamic and structural properties of binary calcium silicate glasses: insights from molecular dynamics
Abstract
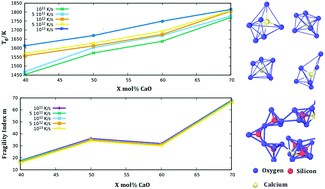
We study calcium silicate glass of composition (CaO)X(SiO2)(1−X), where X = 40–70 mol%, by means of molecular dynamics for different cooling rates between 1011–1013 K s−1. The thermodynamic and kinetic properties of calcium silicate materials are determined, discussed, and correlated to local structures at short and intermediate range orders and to the potential energies of the oxygen atoms. We show that the amount of non-bridging oxygens and the appearance of free oxygens are related to the increase of the glass transition temperature for an increasing CaO content. Our results are analyzed and discussed in connection with the available experimental data.


 Please wait while we load your content...
Please wait while we load your content...