Surface configuration and wettability of nickel(oxy)hydroxides: a first-principles investigation
Abstract
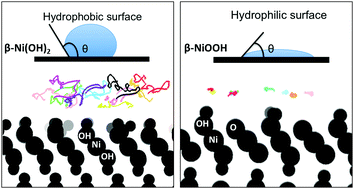
This article explores the wetting behavior of β-type nickel hydroxide, β-Ni(OH)2, and nickel oxyhydroxide, β-NiOOH, by means of first-principles calculations. Water is found to interact weakly with β-Ni(OH)2(001), but strongly with β-NiOOH(001). As unveiled with the use of ab initio molecular dynamics simulations, surface water layers at β-NiOOH(001) show a high degree of ordering correlated with a large surface polarization effect. In comparison, interfacial water at β-Ni(OH)2(001) exhibits enhanced disorder and higher mobility. The weak interaction of water with β-Ni(OH)2(001) is consistent with the small dipole moment of this surface. On the surface of β-NiOOH(001), in addition to the significantly increased surface dipole moment, unsaturated O atoms increase the number of hydrogen bonds between water molecules and the surface, resulting in strong water binding. The wettability trends found in this simulation study are consistent with experimental observations. Another theoretical observation is the increased work function of β-NiOOH(001) relative to β-Ni(OH)2(001) that agrees with experimental results reported in the literature.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...