On the combustion mechanisms of ZrH2 in double-base propellant†
Abstract
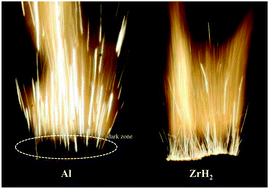
Metal hydrides are regarded as a series of promising hydrogen-supplying fuel for solid rocket propellants. Their effects on the energetic and combustion performances of propellants are closely related to their reaction mechanisms. Here we report a first attempt to determine the reaction mechanism of ZrH2, a high-density metal hydride, in the combustion of a double-base propellant to evaluate its potential as a fuel. ZrH2 is determined to possess good resistance to oxidation by nitrocellulose and nitroglycerine. Thus its combustion starts with dehydrogenation to generate H2 and metallic Zr. Subsequently, the newly formed Zr and H2 participate in the combustion and, especially, Zr melts and then combusts on the burning surface which favors the heat feedback to the propellant. This phenomenon is completely different from the combustion behavior of the traditional fuel Al, where the Al particles are ejected off the burning surface of the propellant to get into the luminous flame zone to burn. The findings in this work validate the potential of ZrH2 as a hydrogen-supplying fuel for double-base propellants.


 Please wait while we load your content...
Please wait while we load your content...