Enhanced conductivity of sodium versus lithium salts measured by impedance spectroscopy. Sodium cobaltacarboranes as electrolytes of choice†
Abstract
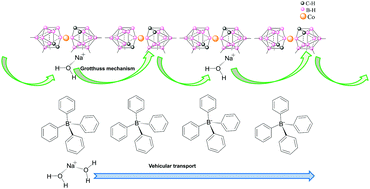
The development of new types of ion conducting materials is one of the most important challenges in the field of energy. Lithium salt polymer electrolytes have been the most convenient, and thus the most widely used in the design of the new generation of batteries. However, in this work, we have observed that Na+ ions provide a higher conductivity, or at least a comparable conductivity to that of Li+ ions in the same basic material. This provides an excellent possibility to use Na+ ions in the design of a new generation of batteries, instead of lithium, to enhance conductivity and ensure wide supply. Our results indicate that the dc-conductivity is larger when the anion is [Co(C2B9H11)2]−, [COSANE]−, compared to tetraphenylborate, [TPB]−. Our data also prove that the dc-conductivity behavior of Li+ and Na+ salts is opposite with the two anions. At 40 °C, the conductivity values change from 1.05 × 10−2 S cm−1 (Li[COSANE]) and 1.75 × 10−2 S cm−1 (Na[COSANE]) to 2.8 × 10−3 S cm−1 (Li[TPB]) and 1.5 × 10−3 S cm−1 (Na[TPB]). These findings indicate that metallacarboranes can be useful components of mixed matrix membranes (MMMs), providing excellent conductivity when the medium contains sufficient amounts of ionic components and a certain degree of humidity.


 Please wait while we load your content...
Please wait while we load your content...