A promising single atom catalyst for CO oxidation: Ag on boron vacancies of h-BN sheets†
Abstract
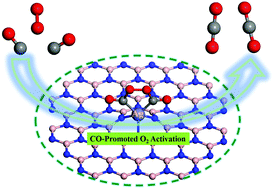
Single atom catalysts (SACs) have attracted broad research interest in recent years due to their importance in various fields, such as environmental protection and energy conversion. Here, we discuss the mechanisms of CO oxidation to CO2 over single Ag atoms supported on hexagonal boron-nitride sheets (Ag1/BN) through systematic van der Waals inclusive density functional theory (DFT-D) calculations. The Ag adatom can be anchored onto a boron defect (VB), as suggested by the large energy barrier of 3.12 eV for Ag diffusion away from the VB site. Three possible mechanisms (i.e., Eley–Rideal, Langmuir–Hinshelwood, and termolecular Eley–Rideal) of CO oxidation over Ag1/BN are investigated. Due to “CO-Promoted O2 Activation”, the termolecular Eley–Rideal (TER) mechanism is the most relevant one for CO oxidation over Ag1/BN and the rate-limiting reaction barrier is only 0.33 eV. More importantly, the first principles molecular dynamics simulations confirm that CO oxidation via the TER mechanism may easily occur at room temperature. Analyses with the inclusion of temperature and entropy effects further indicate that the CO oxidation via the TER mechanism over Ag1/BN is thermodynamically favorable in a broad range of temperatures.


 Please wait while we load your content...
Please wait while we load your content...