Cation distribution and vacancies in nickel cobaltite†
Abstract
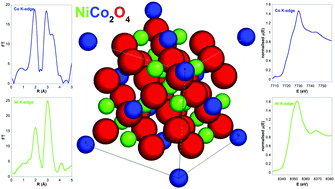
Samples of nickel cobaltite, a mixed oxide occurring in the spinel structure which is currently extensively investigated because of its prospective application as ferromagnetic, electrocatalytic, and cost-effective energy storage material were prepared in the form of nanocrystals stabilized in a highly porous silica aerogel and as unsupported nanoparticles. Nickel cobaltite nanocrystals with average size 4 nm are successfully grown for the first time into the silica aerogel provided that a controlled oxidation of the metal precursor phases is carried out, consisting in a reduction under H2 flow followed by mild oxidation in air. The investigation of the average oxidation state of the cations and of their distribution between the sites within the spinel structure, which is commonly described assuming the Ni cations are only located in the octahedral sites, has been carried out by X-ray absorption spectroscopy providing evidence for the first time that the unsupported nickel cobaltite sample has a Ni : Co molar ratio higher than the nominal ratio of 1 : 2 and a larger than expected average overall oxidation state of the cobalt and nickel cations. This is achieved retaining the spinel structure, which accommodates vacancies to counterbalance the variation in oxidation state.


 Please wait while we load your content...
Please wait while we load your content...