Investigation of the relationship between the cycle performance and the electronic structure in LiAlxMn2−xO4 (x = 0 and 0.2) using soft X-ray spectroscopy†
Abstract
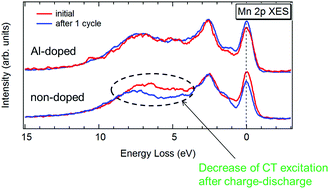
Al doping into LiMn2O4 is one of the well-known methods to improve the cycle performance of the LiMn2O4 cathode. We carried out soft X-ray emission spectroscopy (XES) for LiMn2O4 and LiAl0.2Mn1.8O4 to elucidate the relationship between the Mn 3d electronic structures and cycle performances. After the first cycle, the XES spectra of LiAl0.2Mn1.8O4 are almost unchanged compared to the initial state. In contrast, charge-transfer excitation for the XES of LiMn2O4 is significantly reduced, indicating that the Mn 3d-O 2p hybridization in LiMn2O4 should be easily weakened by charge–discharge. In LiAl0.2Mn1.8O4, the Mn–O bond becomes more stable due to the decrease of Mn3+ ions with Jahn–Teller distortion by Al3+ doping, resulting in the improved cycle performance.


 Please wait while we load your content...
Please wait while we load your content...