3D porous ZnO–SnS p–n heterojunction for visible light driven photocatalysis†
Abstract
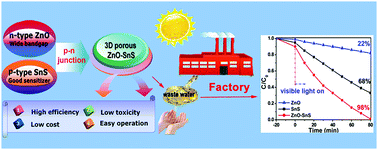
A novel two-step solution approach is put forward to design a unique three dimensional (3D) porous ZnO–SnS p–n heterojunction under mild conditions. This special 3D structure is induced via flower-like ZnO in which SnS serves as an efficient photosensitizer to improve the light harvesting across the whole visible range. A profound investigation of the mechanism shows that this 3D porous ZnO–SnS material effectively integrates the large surface area and high redox potential of ZnO, and wide visible-light harvesting of SnS, which largely promotes the transfer and separation rate of carriers. The systematic study on the active species generated during the photocatalysis illustrates that it is the photoelectrons, ˙OH and O2˙− that play the crucial role in the degradation of dyes. As a result, the noble-metal free photocatalyst degrades nearly 100% of rhodamine B (RhB) within 80 min and methylene blue (MB) in 40 min under visible light. The photocatalytic activity is 10 times higher than that of the pure flower-like ZnO and two times higher than that of the SnS material. Moreover, the photocatalyst is easily separated and reused at least four times without obvious change in efficiency and properties. This work provides an effective strategy for the synthesis of 3D porous p–n heterojunction semiconductor-based photocatalysts with low cost and low toxicity, which present promising applications in the field of solar energy storage and conversion.


 Please wait while we load your content...
Please wait while we load your content...