Aggregation of cyclic polypeptoids bearing zwitterionic end-groups with attractive dipole–dipole and solvophobic interactions: a study by small-angle neutron scattering and molecular dynamics simulation†
Abstract
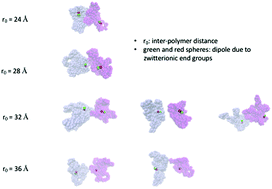
Aggregation behavior of cyclic polypeptoids bearing zwitterionic end-groups in methanol has been studied using a combination of experimental and simulation techniques. The data from SANS and cryo-TEM indicate that the solution contains small clusters of these cyclic polypeptoids, ranging from a single polypeptoid chain to small oligomers, while the linear counterpart shows no cluster formation. Atomistic molecular dynamics simulations reveal that the driving force for this clustering behavior is due to the interplay between the effective repulsion due to the solvation of the dipoles formed by the charged end-groups in each polypeptoid chain and the attractive forces due to dipole–dipole interactions and the solvophobic effect.


 Please wait while we load your content...
Please wait while we load your content...