Femtosecond to nanosecond studies of octupolar molecules and their quadrupolar and dipolar analogues†
Abstract
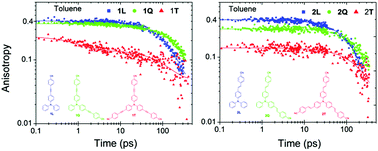
The photophysical properties of two octupolar (T) molecules and of their linear (L) and quadrupolar (Q) analogues are studied by means of steady state and femtosecond to nanosecond spectroscopy. The compounds bear a triphenylamine donor, cyano acceptors and acetylenic (series 1) or olefin (series 2) π-bridges. In the octupolar compound of series 2 (2T), fluorescence is emitted from an excited state localized on a single branch, while in that of series 1 (1T), the emitting state is delocalized among branches pointing to a reduced excited state polarity. Excited state dynamics in series 1 has shown an increase of lifetime with solvent polarity. In the branched compounds of series 2, multiexponential dynamics in polar solvents is exhibited indicating a distribution of emitting geometries. Femtosecond anisotropy in 1T indicates incoherent excitation transfer on the timescale of a few ps, in agreement with the hopping time predicted by the Förster model. However, no hopping mechanism is observed in 2T possibly because of an increased intramolecular charge transfer leading to a low energy relaxed excited state localized on a single branch.


 Please wait while we load your content...
Please wait while we load your content...