Mechanisms of H and CO loss from the uracil nucleobase following low energy electron irradiation†
Abstract
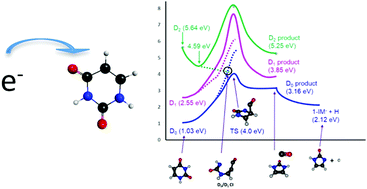
Ionising radiation in cells generates secondary low energy electrons (LEEs) which can induce biomolecular damage when incident upon a particular biomolecule. Notable biomolecules include those contained within double-stranded DNA and RNA helices, which upon exposure to LEEs, may form reactive intermediate products that show detriment to their specific structures and functions. Such damaging processes are understood to proceed via dissociative electron attachment (DEA). Using anion resonance stabilisation methods, coupled to state-of-the-art electronic structure methods, and reaction path mapping, the work presented herein highlights the detailed mechanisms associated with molecular elimination of CO and H from the uracil nucleobase following LEE irradiation – providing a rationale for earlier experiments. The results highlight a subset of resonances that show a labile route for forming CO + anionic 1,3-dihydroimidazole (1,3-DHIM−). The nascent 1,3-DHIM anion is itself unstable with respect to N–H bond fission – ultimately forming anionic 1-imidazole (1-IM−) + H products. This hitherto unknown mechanism sheds light on the possible lesion processes that may be encountered by biomolecular constituents when exposed to electron energies analogous to those present in medicinal X-ray diagnostics, aviation and interstellar space. The work also offers some insight into the reactive processes that may have occurred in prebiotic earth.


 Please wait while we load your content...
Please wait while we load your content...