Electron-triggered chemistry in HNO3/H2O complexes†
Abstract
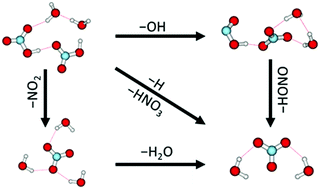
Polar stratospheric clouds, which consist mainly of nitric acid containing ice particles, play a pivotal role in stratospheric chemistry. We investigate mixed nitric acid–water clusters (HNO3)m(H2O)n, m ≈ 1–6, n ≈ 1–15, in a laboratory molecular beam experiment using electron attachment and mass spectrometry and interpret our experiments using DFT calculations. The reactions are triggered by the attachment of free electrons (0–14 eV) which leads to subsequent intracluster ion–molecule reactions. In these reactions, the nitrate anion NO3− turns out to play the central role. This contradicts the electron attachment to the gas-phase HNO3 molecule, which leads almost exclusively to NO2−. The nitrate containing clusters are formed through at least three different reaction pathways and represent terminal product ions in the reaction cascade initiated by the electron attachment. Besides, the complex reaction pathways represent a new hitherto unrecognized source of atmospherically important OH and HONO molecules.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...