Local chemical potential, local hardness, and dual descriptors in temperature dependent chemical reactivity theory
Abstract
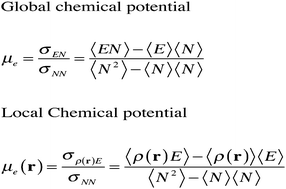
In this work we establish a new temperature dependent procedure within the grand canonical ensemble, to avoid the Dirac delta function exhibited by some of the second order chemical reactivity descriptors based on density functional theory, at a temperature of 0 K. Through the definition of a local chemical potential designed to integrate to the global temperature dependent electronic chemical potential, the local chemical hardness is expressed in terms of the derivative of this local chemical potential with respect to the average number of electrons. For the three-ground-states ensemble model, this local hardness contains a term that is equal to the one intuitively proposed by Meneses, Tiznado, Contreras and Fuentealba, which integrates to the global hardness given by the difference in the first ionization potential, I, and the electron affinity, A, at any temperature. However, in the present approach one finds an additional temperature-dependent term that introduces changes at the local level and integrates to zero. Additionally, a τ-hard dual descriptor and a τ-soft dual descriptor given in terms of the product of the global hardness and the global softness multiplied by the dual descriptor, respectively, are derived. Since all these reactivity indices are given by expressions composed of terms that correspond to products of the global properties multiplied by the electrophilic or nucleophilic Fukui functions, they may be useful for studying and comparing equivalent sites in different chemical environments.


 Please wait while we load your content...
Please wait while we load your content...