The effect of substituents on triply bonded boron![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) antimony molecules: a theoretical approach†
antimony molecules: a theoretical approach†
Abstract
Three (M06-2X/Def2-TZVP, B3PW91/Def2-TZVP and B3LYP/LANL2DZ+dp) levels of theory are used to study the effect of substituents on the potential energy surfaces of RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR (R = F, OH, H, CH3, SiH3, SiMe(SitBu3)2, SiiPrDis2 and NHC). The theoretical results demonstrate that the triply bonded RB
SbR (R = F, OH, H, CH3, SiH3, SiMe(SitBu3)2, SiiPrDis2 and NHC). The theoretical results demonstrate that the triply bonded RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules favor a bent geometry: that is, ∠R–B–Sb ≈ 180° and ∠B–Sb–R ≈ 120°. Regardless of the type of substituents that are attached to the RB
SbR molecules favor a bent geometry: that is, ∠R–B–Sb ≈ 180° and ∠B–Sb–R ≈ 120°. Regardless of the type of substituents that are attached to the RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR compounds, theoretical evidence strongly indicates that their B
SbR compounds, theoretical evidence strongly indicates that their B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Sb triple bonds have a donor–acceptor nature and are proven to be very weak. Two valence bond models clarify the bonding characters of the B
Sb triple bonds have a donor–acceptor nature and are proven to be very weak. Two valence bond models clarify the bonding characters of the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Sb triple bond. For RB
Sb triple bond. For RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules that feature small substituents, the triple bond is represented as
SbR molecules that feature small substituents, the triple bond is represented as 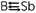 . For RB
. For RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules that feature large substituents, the triple bond is represented as
SbR molecules that feature large substituents, the triple bond is represented as  . Most importantly, this theoretical study predicts that only bulkier substituents significantly stabilize the triply bonded RB
. Most importantly, this theoretical study predicts that only bulkier substituents significantly stabilize the triply bonded RB![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules, from the kinetic viewpoint.
SbR molecules, from the kinetic viewpoint.
![Graphical abstract: The effect of substituents on triply bonded boron [[triple bond, length as m-dash]] antimony molecules: a theoretical approach](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CP00421D&imageInfo.ImageIdentifier.Year=2017)


 Please wait while we load your content...
Please wait while we load your content...
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) antimony molecules: a theoretical approach
antimony molecules: a theoretical approach