Direct observation of the rise of delayed fluorescence in dithienylbenzothiadiazole and its role in the excited state dynamics of a donor–acceptor–donor molecule†
Abstract
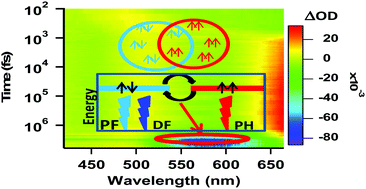
Dithienylbenzothiadiazole (DTBT) is used as a building block in several molecules having application in organic light emitting devices (OLED) and organic photovoltaic (OPV) devices. Delayed fluorescence (DF) is the preferred design principle employed currently in OLED research. DTBT has excellent delayed fluorescence properties which makes this moiety a potentially viable OLED material. Here, the dynamics of intersystem crossing (ISC) and reverse intersystem crossing (RISC) have been explored using fluorescence, phosphorescence, fluorescence lifetime and transient absorption measurements. Experimentally it is demonstrated that singlet and triplet states of DTBT are close lying or degenerate and after a certain time the molecules can stay in the singlet or the triplet state forming an equilibrium between the two states which hinders the identification of these two states that could be characterized by routine steady state fluorescence and phosphorescence studies. Similarly in OPV material research, DTBT is coupled with strong donors to form push–pull molecules to produce a prolonged charge separated state. In this study DTBT appended with carbazole at both ends (CDTBT) was used to study the dynamics of DTBT within a donor–acceptor–donor system. The study reveals similar kinds of ISC and RISC in CDTBT along with a competitive deactivation pathway of the singlet state and it was concluded to be through the formation of a charge separated species in CDTBT.


 Please wait while we load your content...
Please wait while we load your content...