Tuning the oxidative power of free iron–sulfur clusters
Abstract
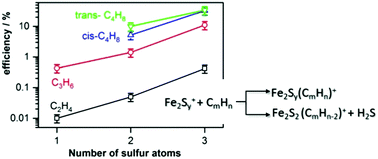
The gas-phase reactions between a series of di-iron sulfur clusters Fe2Sx+ (x = 1–3) and the small alkenes C2H4, C3H6, and C4H8 have been investigated by means of Fourier-transform ion-cyclotron resonance mass spectrometry. For all studied alkenes, the reaction efficiency is found to increase in the order Fe2S+ < Fe2S2+ < Fe2S3+. In particular, Fe2S+ and Fe2S2+ only form simple association products, whereas the sulfur-rich Fe2S3+ is able to dehydrogenate propene and 2-butene via desulfurization of the cluster and formation of H2S. This indicates an increased propensity to induce oxidation reactions, i.e. oxidative power, of Fe2S3+ that is attributed to an increased formal oxidation state of the iron atoms. Furthermore, the ability of Fe2S3+ to activate and dissociate the C–H bonds of the alkenes is observed to increase with increasing size of the alkene and thus correlates with the alkene ionization energy.


 Please wait while we load your content...
Please wait while we load your content...