Evaporation induced nanoparticle – binder interaction in electrode film formation†
Abstract
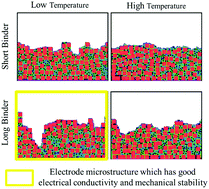
Processing induced nanoparticle agglomeration and binder distribution affect the electrode microstructure formation and corresponding electrochemical performance in lithium-ion batteries. In the present study, stochastic dynamics computations based on a morphologically detailed mesoscale model are performed to illustrate the microstructural variability of electrode films affected by the evaporation condition (drying temperature) and the binder length (molecular weight). Micropores are observed at the surface of the electrode film when dried at a lower temperature. The pore formation depth tends to increase as the binder length increases. The solvent chemical potential also affects the surface topography of the electrode film. The solvent with higher volatility (more negative chemical potential) tends to produce more micropores. A lower drying temperature is beneficial for improving the electronic conductivity of the porous electrode film due to the better distribution of the conductive additive nanoparticles on and around the active particles, thereby facilitating the electron transport network formation. Agglomeration between active material nanoparticles can also be mitigated at a lower drying temperature. Additionally, better adhesion of the porous electrode film can be achieved due to preferential localization of the binder on the substrate at relatively low-temperature evaporation.


 Please wait while we load your content...
Please wait while we load your content...