Alkaline-earth metal (Mg) polynitrides at high pressure as possible high-energy materials†
Abstract
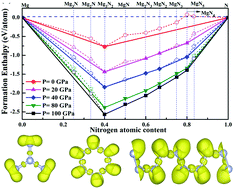
The high-pressure structural evolutionary behaviors of magnesium polynitrides were studied up to 100 GPa using first-principles calculations. Using the unbiased structure searching method, five stable chemical stoichiometries of magnesium polynitrides (MgN, Mg2N3, MgN2, MgN3, and MgN4) were theoretically predicted at high pressures. The predicted MgNx compounds contain a rich variety of polynitrogen forms ranging from charged molecules (one-dimensional bent molecules N3, planar triangle N4 to benzene-like rings N6) to extended polymeric chains (N∞). To the best of our knowledge, this is the first time that stable bent molecules N3, planar triangle N4, and polymeric chains (N∞) were predicted in alkaline-earth metal polynitrides. The decomposition of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) -MgN3 and P
-MgN3 and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) -MgN4 are expected to be highly exothermic, releasing an energy of approximately 2.83 kJ g−1 and 2.01 kJ g−1, respectively. Furthermore, P
-MgN4 are expected to be highly exothermic, releasing an energy of approximately 2.83 kJ g−1 and 2.01 kJ g−1, respectively. Furthermore, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) -MgN4 can be synthesized at several GPa. The results of the present study suggest that it is possible to obtain energetic polynitrogen in main-group nitrides under high pressure.
-MgN4 can be synthesized at several GPa. The results of the present study suggest that it is possible to obtain energetic polynitrogen in main-group nitrides under high pressure.


 Please wait while we load your content...
Please wait while we load your content...