The secret behind the success of doping nickel oxyhydroxide with iron†
Abstract
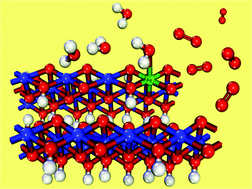
Discovering better catalysts for water splitting is the holy grail of the renewable energy field. One of the most successful water oxidation catalysts is nickel oxyhydroxide (NiOOH), which is chemically active only as a result of doping with Fe. In order to shed light on how Fe improves efficiency, we perform Density Functional Theory +U (DFT+U) calculations of water oxidation reaction intermediates of Fe substitutional doped NiOOH. The results are analyzed while considering the presence of vacancies that we use as probes to test the effect of adding charge to the surface. We find that the smaller electronegativity of the Fe dopant relative to Ni allows the dopant to have several possible oxidation states with less energy penalty. As a result, the presence of vacancies which alters local oxidation states does not affect the low overpotential of Fe-doped NiOOH. We conclude that the secret to the success of doping NiOOH with iron is the ability of iron to easily change oxidation states, which is critical during the chemical reaction of water oxidation.


 Please wait while we load your content...
Please wait while we load your content...