Swollen micelles and alcohol–surfactant co-adsorption: structures and mechanisms from liquid- and solid-state 1H–1H NMR spectroscopy†
Abstract
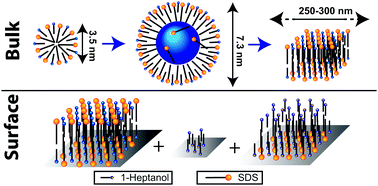
Mixtures of surfactants and medium chained alcohols display an anomalous phase behaviour, with the formation of swollen micelles in mid-range surfactant concentrations, which transition into larger non-swollen aggregates when the surfactant concentration increases above a critical point. These alcohols also affect the adsorption behaviour of the surfactants. In this study, intermolecular proximities are measured for such systems by 1H–1H NMR dipolar correlation experiments, giving molecular localizations. The medium chained 1-heptanol and an anionic surfactant sodium dodecyl sulphate (SDS) are studied, both solubilized and adsorbed on alumina. Nuclear Overhauser Effect Spectroscopy (NOESY) shows that 1-heptanol localizes in both the palisade layer and in the core of SDS micelles when the 1-heptanol : SDS mole ratio increases beyond 2. The micelle diameter then increases with increasing 1-heptanol : SDS mole ratios due to more 1-heptanol partitioning in the micelle interior. When the micelle diameter increases beyond ∼6 nm, some SDS moves into the micelle interior, which may be a driving force for the structural transition at higher SDS concentrations. After being adsorbed on alumina, 1H–1H double-quantum magic angle spinning (DQ MAS) shows that SDS/1-heptanol bilayers are formed where 1-heptanol localizes in the palisade layer only, but with slightly different localizations compared to that in micelles. Three different 1-heptanol environments are identified on the surface by 2H NMR using 2H labelled 1-heptanol. However, in contrast to in solution, no 1-heptanol adsolubilizes in the bilayer interior.


 Please wait while we load your content...
Please wait while we load your content...