Catalytic decomposition and mechanism of formaldehyde over Pt–Al2O3 molecular sieves at room temperature
Abstract
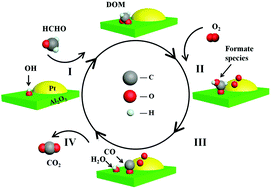
To overcome the drawbacks of powder-like catalysts in practical application, an Al2O3 molecular sieve supported Pt (Pt–Al2O3) catalyst was prepared by the impregnation method combined with dilute hydrochloric acid solution pretreatment. The role of the pretreatment solution in catalyst preparation and formaldehyde (HCHO) decomposition over Pt–Al2O3 catalysts was investigated. The acid solution with pH = 1 was optimal for Pt dispersion on the Al2O3 surface. The Pt–Al2O3 catalyst was capable of achieving complete and stable HCHO oxidation at ambient temperature. In addition, the excellent activity for HCHO removal was promoted by the mesoporous structure, large specific surface area and high adsorption ability of Al2O3 molecular sieves, as determined by N2-sorption studies. Dioxymethylene (DOM) and formate species were identified as major intermediates by in situ DRIFTS studies. These intermediates were subsequently oxidized into CO2 and H2O at ambient temperature. Moreover, a possible reaction mechanism of HCHO removal and a regeneration pathway of surface hydroxyls were proposed. This study provides new insights into the preparation of practical, low-cost catalysts for indoor air purification.


 Please wait while we load your content...
Please wait while we load your content...