The reaction mechanism of sarcosine oxidase elucidated using FMO and QM/MM methods†
Abstract
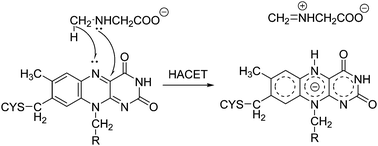
Monomeric sarcosine oxidase (MSOX) is a flavoprotein that oxidizes sarcosine to the corresponding imine product and is widely used in clinical diagnostics to test renal function. In the past decade, several experimental studies have been performed to elucidate the underlying mechanism of this oxidation reaction. However, the details of the molecular mechanism remain unknown. In this study, we theoretically examined three possible reaction mechanisms, namely, the single-electron transfer, hydride-transfer, and polar mechanisms, using the fragment molecular orbital (FMO) and mixed quantum mechanics/molecular mechanics (QM/MM) methods. We found that, of the three possible reaction pathways, hydride-transfer is the most energetically favorable mechanism. Significantly, hydrogen is not transferred in the hydride state (H−) but in a hydrogen atom state (H˙). Furthermore, a single electron is simultaneously transferred from sarcosine to flavin through their overlapping orbitals. Therefore, based on a detailed theoretical analysis of the calculated reaction pathway, the reaction mechanism of MSOX can be labeled the “hydrogen-atom-coupled electron-transfer” (HACET) mechanism instead of being categorized as the classical hydride-transfer mechanism. QM/MM and FMO calculations revealed that sarcosine is moved close to the flavin ring because of a small charge transfer (about 0.2 electrons in state 1 (MSOX–sarcosine complex)) and that the positively charged residues (Arg49, Arg52, and Lys348) near the active site play a prominent role in stabilizing the sarcosine–flavin complex. These results indicate that strong Coulombic interactions primarily control amine oxidation in the case of MSOX. The new reaction mechanism, HACET, will be important for all the flavoprotein-catalyzed oxidation reactions.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...