Deep eutectic solvents: similia similibus solvuntur?†
Abstract
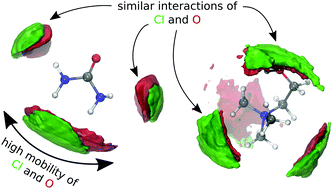
Deep eutectic solvents, mixtures of an organic compound and a salt with a deep eutectic melting point, are promising cheap and eco-friendly alternatives to ionic liquids. Ab initio molecular dynamics simulations of reline, a mixture consisting of urea and choline chloride, reveal that not solely hydrogen bonds allow similar interactions between both constituents. The chloride anion and the oxygen atom of urea also show a similar spatial distribution close to the cationic core of choline due to a similar charge located on both atoms. As a result of multiple similar interactions, clusters migrating together cannot be observed in reline which supports the hypothesis similia similibus solvuntur. In contrast to previous suggestions, the interaction of the hydroxyl group of choline with a hydrogen bond acceptor is overall rigid. Fast hydrogen bond acceptor dynamics is facilitated by the hydrogen atoms in the trans position to the carbonyl group of urea which contributes to the low melting point of reline.


 Please wait while we load your content...
Please wait while we load your content...