Identifying the optimal anticancer targets from the landscape of a cancer–immunity interaction network†
Abstract
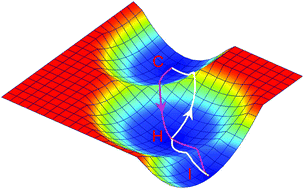
Cancer immunotherapy, an approach of targeting immune cells to attack tumor cells, has been suggested to be a promising way for cancer treatment recently. However, the successful application of this approach warrants a deeper understanding of the intricate interplay between cancer cells and the immune system. Especially, the mechanisms of immunotherapy remain elusive. In this work, we constructed a cancer–immunity interplay network by incorporating interactions among cancer cells and some representative immune cells, and uncovered the potential landscape of the cancer–immunity network. Three attractors emerge on the landscape, representing the cancer state, the immune state, and the hybrid state, which can correspond to escape, elimination, and equilibrium phases in the immunoediting theory, respectively. We quantified the transition processes between the cancer state and the immune state by calculating transition actions and identifying the corresponding minimum action paths (MAPs) between these two attractors. The transition actions, directly calculated from the high dimensional system, are correlated with the barrier heights from the landscape, but provide a more precise description of the dynamics of a system. By optimizing the transition actions from the cancer state to the immune state, we identified some optimal combinations of anticancer targets. Our combined approach of the landscape and optimization of transition actions offers a framework to study the stochastic dynamics and identify the optimal combination of targets for the cancer–immunity interplay, and can be applied to other cell communication networks or gene regulatory networks.


 Please wait while we load your content...
Please wait while we load your content...