Massive dihydrogen uptake by anionic carbon chains†
Abstract
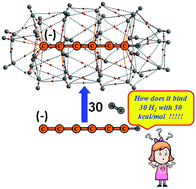
Acetylene and polyyne carbon chains show a negligible ability to bind even a single dihydrogen molecule. M06L/6-311++G(d,p) DFT and CCSD(T)/aug-cc-pVTZ//CCSD/aug-cc-pVDZ calculations corroborate that these carbon chains in the first deprotonated anionic and second deprotonated dianionic forms display massive dihydrogen uptake capabilities (45.3 to 62.8 wt%). The coordinatively saturated complexes of these anions and dianions with chain lengths of up to six carbons hold 20–32 H2 molecules. The interaction energy (Eint) values of the saturated state of the monoanions (44.5–50.0 kcal mol−1) and dianions (79.8–87.4 kcal mol−1) indicate substantial energetic stabilization per H2 molecule adsorbed. The binding of H2 to the carbon chain is established by the observation of bond critical points in the electron density analysis. The noncovalently bonded interconnections of the adsorbed H2 molecules indicated by H2⋯H2 bond critical points, provide additional stability to the complex keeping the system a fully noncovalently allied entity. Further, molecular electrostatic potential analysis (MESP) is conducted to study the delocalization of the extra electron(s) over the entire complex.


 Please wait while we load your content...
Please wait while we load your content...