Pressure-driven phase transition mechanisms revealed by quantum chemistry: l-serine polymorphs†
Abstract
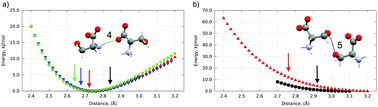
The present study delivers a computational approach for the understanding of the mechanism of phase transitions between polymorphs of small organic molecules. By using state of the art periodic DFT calculations augmented with dispersion corrections and an external stress tensor together with gas-phase cluster calculations, we thoroughly explained the reversible phase transitions of three polymorphs of the model system, namely crystalline L-serine in the pressure range up to 8 GPa. This study has shown that at the macroscopic level the main driving force of the phase transitions is the decrease in the volume of the crystal unit cell, which contributes to the enthalpy difference between the two forms, but not to the difference in their internal crystal energies. At the microscopic level we suggest that hydrogen bond overstrain leads to a martensitic-like, cooperative, displacive phase transition with substantial experimental hysteresis, while no such overstrain was found for the “normal type”, atom per atom, reconstructive phase transition. The predicted pressures for the phase transitions deducted by the minimum enthalpy criterion are in reasonable agreement with the observed ones. By delivering unambiguous explanations not provided by previous studies and probably not accessible to experiment, this work demonstrates the predictive and explanatory power of quantum chemistry, confirming its indispensable role in structural studies.


 Please wait while we load your content...
Please wait while we load your content...