Beyond the electrostatic model: the significant roles of orbital interaction and the dispersion effect in aqueous–π systems†
Abstract
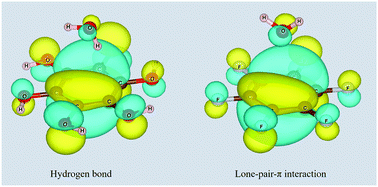
The electrostatic model, which is widely used to explain π-involved interactions, gives an intuitive picture of these intermolecular interactions and has successfully predicted many phenomena in recent decades. Still, this simple model faces problems in certain cases and it has come under fire in previous studies on π–π stacking interactions. Here, employing ab initio calculations, we have identified several counter examples in aqueous–π systems which cannot be explained adequately using the classical electrostatic model, and revealed the underlying reason. We find strong intermolecular orbital interactions in both aqueous–π hydrogen bond and lone-pair–π interactions, and thus extend the previously proposed new model for π–π stacking to aqueous–π systems: while the electrostatic attraction and dispersion effects are the main driving forces pulling the monomers together, Pauli repulsion plays a role in keeping them apart. Interestingly, the molecular orbitals on the concerned monomers exhibit a weak bonding nature at the equilibrium distance.


 Please wait while we load your content...
Please wait while we load your content...