Synthesis and characterization of asymmetrical gemini surfactants
Abstract
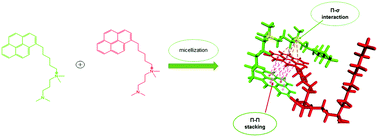
The effect of variation in the length of surfactant hydrocarbon tail groups was tested in a series of dissymmetric gemini surfactants (N1-alkyl N1,N1,N3,N3-tetramethyl-N3-(6-pyren-6yl)-hexyl)propane-1,3-diammonium dibromide designated as CmC3CnBr, with m = hexyl pyrene, and n = 8, 12, 14, 16, and 18. The aggregation properties of these surfactants have been investigated by means of 1H NMR, fluorescence spectroscopy, surface tension and electrical conductivity measurements. The critical micelle concentration (CMC) was determined using surface tension and confirmed using the specific conductance method. Krafft temperatures and the degree of micelle ionization (α) were obtained from specific conductance measurements. With an increase of the dissymmetry (m/n) ratio, the CMC decreased linearly and an increase in the Krafft temperatures was observed for all of the gemini surfactants. α values for the dissymmetric GS were higher than those of the m-3-m counterparts, which may be attributed to enhanced micelle–micelle interactions that arise from increased hydrophobicity of the hydrocarbon chains. The introduction of the bulky pyrenyl tail group resulted in much lower CMC values compared to their symmetrical counterparts affecting the packing of these surfactants at the air/water interface, which resulted in high-ordered structures (lamellar and inverted micelles). This in turn affected the thermodynamic parameters of the micellization.


 Please wait while we load your content...
Please wait while we load your content...