Introducing a closed system approach for the investigation of chemical steps involving proton and electron transfer; as illustrated by a copper-based water oxidation catalyst†
Abstract
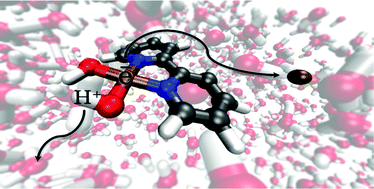
The investigation of the catalytic mechanism of homogeneous water oxidation catalysts remains an active field of research. When examining catalytic steps theoretically, it is often difficult to account for the transfer of protons and electrons from step to step. To this end, a closed system approach is proposed which includes both proton and electron acceptors in the simulation box to allow for the description of proton-coupled electron transfer processes. Using Car–Parrinello Molecular Dynamics, a mononuclear copper water oxidation catalyst Cu(bpy)(OH)2 was used as a model system to explore this closed system approach. The exploration of this model system shows that, compared to traditional methods, this approach offers extra insight into proposed catalytic steps and allows for the clear identification of preferred reaction paths.
- This article is part of the themed collection: Developments in Density Functional Theory


 Please wait while we load your content...
Please wait while we load your content...