Photoinduced electron transfer in layer-by-layer thin solid films containing cobalt oxide nanosheets, porphyrin, and methyl viologen†
Abstract
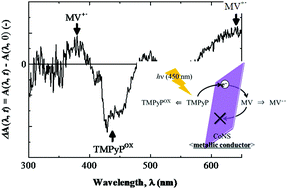
The well-known layer-by-layer (LbL) method can be used to prepare solid thin films with a controlled electron transfer direction by appropriately stacking metal oxide nanosheets and functional organic ions. In this study, we prepared thin solid films consisting of cobalt oxide nanosheets (CoNSs) as the electron transfer medium, α,β,γ,δ-tetrakis(1-methylpyridinium-4-yl)porphyrin (TMPyP) as the electron donor, and 1,1′-dimethyl-4,4′-bipyridinium or methyl viologen (MV) as the electron acceptor. We investigated the photoinduced electron transfer phenomenon in these films by irradiating them with 450 nm light. Irradiating the LbL thin solid films prepared with the CoNS/TMPyP/CoNS/MV/CoNS sequence under reduced pressure led to the production of a one-electron reduction compound of MV. Hence, photoinduced electron transfer from TMPyP to MV bound to CoNSs occurred in these LbL thin solid films. However, the conduction band of CoNSs, as determined by the photoabsorption spectral and photoelectrochemical measurements, was much higher than the lowest unoccupied molecular orbital level of TMPyP. Our findings indicate that the observed equipotential photoinduced electron transfer was caused by the metallic electron conductivity of CoNSs, which show a unique charge arrangement of Co3+ and Co4+. Moreover, it was also found that the observed photoinduced charge separation state has a longer life-time (>5 h) under the reduced conditions.


 Please wait while we load your content...
Please wait while we load your content...