Unravelling the dissociation pathways of acetic acid upon electron transfer in potassium collisions: experimental and theoretical studies†
Abstract
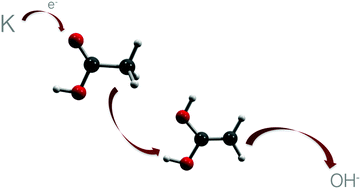
Electron transfer in alkali-molecule collisions with gas phase acetic acid and its deuterated analogues resulting in OH− formation requires considerable internal rearrangement in the temporary negative ion. At a collision energy well above the threshold of negative ion formation, electron transfer from potassium to CH3COOH/CH3COOD and CD3COOH results not only in H transfer from CH3 to COOH/COOD, but also in H release from COOH and subsequent rearrangement to eliminate OH−. These processes are also investigated by theoretical post-Hartree–Fock and DFT calculations. The combination of both studies reveals that the most favourable intermediate mechanism occurs via diol formation. Such intramolecular H transfer is reported here for the first time in the context of electron transfer induced dissociation experiments in alkali-molecule collisions. A comprehensive fragmentation study is presented and dissociation mechanisms are suggested.

 Please wait while we load your content...
Please wait while we load your content...