A systematic study of 25Mg NMR in paramagnetic transition metal oxides: applications to Mg-ion battery materials†
Abstract
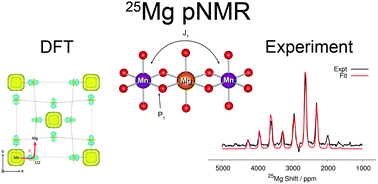
Rechargeable battery systems based on Mg-ion chemistries are generating significant interest as potential alternatives to Li-ion batteries. Despite the wealth of local structural information that could potentially be gained from Nuclear Magnetic Resonance (NMR) experiments of Mg-ion battery materials, systematic 25Mg solid-state NMR studies have been scarce due to the low natural abundance, low gyromagnetic ratio, and significant quadrupole moment of 25Mg (I = 5/2). This work reports a combined experimental 25Mg NMR and first principles density functional theory (DFT) study of paramagnetic Mg transition metal oxide systems Mg6MnO8 and MgCr2O4 that serve as model systems for Mg-ion battery cathode materials. Magnetic parameters, hyperfine shifts and quadrupolar parameters were calculated ab initio using hybrid DFT and compared to the experimental values obtained from NMR and magnetic measurements. We show that the rotor assisted population transfer (RAPT) pulse sequence can be used to enhance the signal-to-noise ratio in paramagnetic 25Mg spectra without distortions in the spinning sideband manifold. In addition, the value of the predicted quadrupolar coupling constant of Mg6MnO8 was confirmed using the RAPT pulse sequence. We further apply the same methodology to study the NMR spectra of spinel compounds MgV2O4 and MgMn2O4, candidate cathode materials for Mg-ion batteries.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...