Abstract
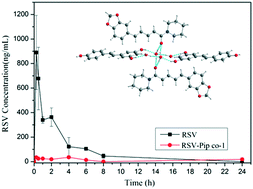
It was reported that the alkaloid component piperine (Pip) can drastically improve the bioavailability of the compound resveratrol (RSV) when co-administered. Therefore, cocrystallization experiments of RSV and Pip were attempted, and four cocrystals are successfully obtained from solution evaporation experiments. Their single crystals are acquired and the structural determination reveals that all the cocrystals contain solvent molecules within the crystals. Confirmed by VT-PXRD, the cocrystals transformed into the amorphous state after desolvation. Considering the toxicity of the solvent molecule, only RSV–Pip co-1 was utilized for further dissolution and pharmacokinetic experiments. The dissolution results demonstrate that RSV–Pip co-1 shows lower dissolution and apparent solubility than the RSV constituent under different buffers in the presence of different surfactants. The reduced dissolution and apparent solubility have a strong diminishing impact on the absorption of RSV, thus leading to unfavorable bioavailability, even though the coformer Pip possesses bioenhancer properties.


 Please wait while we load your content...
Please wait while we load your content...