Nickel(ii) metal–organic frameworks with N,N′-di(4-pyridyl)-naphthalenediimide ligands: influence of secondary building unit geometry on dimensionality and framework dimensions†
Abstract
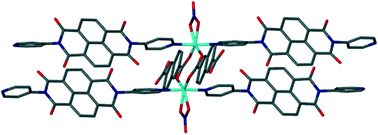
When Ni(NO3)2·6H2O and N,N′-di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide (DPNDI) are reacted, a one-dimensional coordination polymer (1) is formed. However, analogous reactions with either terephthalic acid (2) or 2,6-naphthalenedicarboxylic acid (3) and DPNDI affords two-dimensional, pillared metal–organic frameworks. 2 and 3 contain rectangular voids of different dimensions which are dictated by the carboxylate ligand and the arrangement of the {[M(k2-O2NO)]2(μ2-O2CR)2} secondary building unit (SBU) that forms the nodes of the framework. The role of SBU geometry, intermolecular face-to-face π–π and lone pair–π interactions involving the DPNDI ligands are discussed.


 Please wait while we load your content...
Please wait while we load your content...